Pre-Treatment Considerations
Identifying these aspects of water will help determine the best treatment for filtration, corrosion, and scale issues.
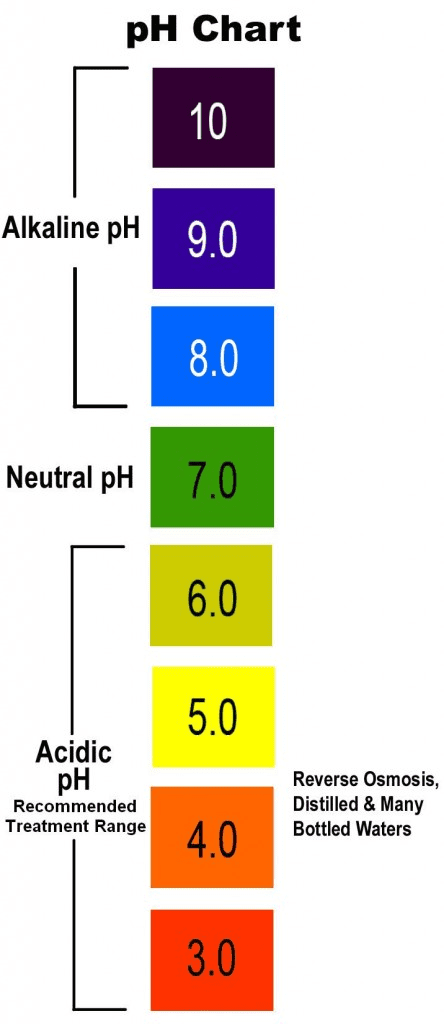
pH vs. Alkalinity
What does the pH measurement of water really tell you as a user? The most common interpretation—"pH tells you how acidic water is"—is broadly correct but hides the important fact that measuring pH does not give you the total amount of acidity present. It only provides an understanding of the acidity present relative to a neutral pH. To better illustrate this concept, think about the relationship between "temperature" and “heat stored in an object.”
For example, a wooden bench and a stone bench are situated along the same path in a sunny park. The wooden bench does not feel too hot, even when the sun has been shining on it all day long, but in contrast, the stone bench, which has been in the same sun just as long – would be an unbearably hot place to sit. Measuring both benches using an infrared thermometer might show them as being the exact same temperature, but the stone bench has a large amount of heat stored (mostly due to its large mass) that it passes to you once you decide (perhaps unwisely) to sit on its surface.
The stone bench – for this same reason – will stay warm for much longer after the sun has set. In short, the stone bench has a higher amount of heat stored (heat capacity) at a given temperature compared to the wooden bench. Therefore, the stone bench reacts with more resistance to temperature change; it has a high inertia.
For water, pH is an indicator of the current state of the water, but it does not contain any information about how easily pH will vary when coffee, gases, or metal boilers come into play. Like the thermometer and the benches, pH does not tell you all the details. With the benches, it was helpful to understand the heat capacity before deciding where to sit. When determining water recipes, alkalinity is that extra bit of information you need; it outlines the water’s resistance to change (or lack thereof). Therefore, pH is to alkalinity what temperature is to the amount of heat stored.
A low pH means the water is acidic, but it does not tell you how much acidity is present, or, to put it another way, how much acidity has gone in to make the pH < 7.
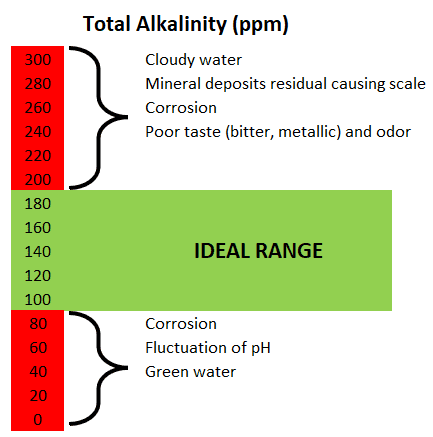
What does this mean for coffee professionals? Effectively, the perceived acidity of a given coffee beverage corresponds to the amount of acid extracted from the coffee minus the amount of alkalinity from the water. It is far more important to keep alkalinity within a certain range than pH when it comes to choosing water for coffee extraction.
Probably the most important and least understood is water. If you are spending money on coffee beans and not spending the time and effort to perfect your water, you may be missing out on many of the hidden flavors still locked in the beans. Water is the canvas for these flavors. The mineral composition and chemistry of the water are as much a determining factor in what you are tasting as the beans themselves.
Recent research has uncovered that it isn’t necessarily the overall total dissolved solids (TDS) but the actual minerals that are dissolved. Christopher Hendon (a Ph.D. student at the University of Bath) and Maxwell Colonna-Dashwood (a World Barista Championship finalist) have studied the chemical composition of water and methods to deliver the best flavor.
“We’ve found that the water composition is key to the proportions of sugars, starches, bases, and acids extracted from a particular roast.”
“Hard water is generally considered bad for coffee, but we found it was the type of hardness that mattered—while high bicarbonate levels are bad, high magnesium ion levels increase the extraction of coffee into water and improve the taste.”
The study also found that sodium-rich water, such as that produced by water softeners, didn’t help the taste of the coffee either.
“There is no one particular perfect composition of water that produces consistently flavorsome extractions from all roasted coffee. But magnesium-rich water is better at extracting coffee compounds, and the resultant flavor depends on the balance between both the ions in the water and the quantity of bicarbonate present."
https://clivecoffee.com/blogs/learn/the-science-of-water-composition-in-espresso
Did you know?
The ideal pH for brewing coffee is a neutral 7, with wiggle room on either side.
More acidic water can enable more delicate flavor compounds to come through, but it results in weak extraction.
More alkaline water facilitates extraction but can suppress flavor. That’s why the middle road is best in the case of pH.



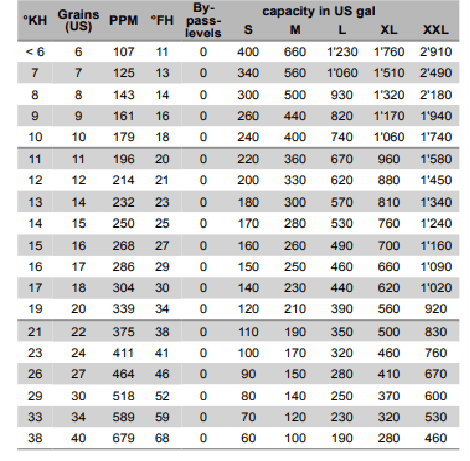
Grains of hardness
Did you know
The higher the grains, the lower the capacity.
The below is from the instructions of a competitor
This illustrates why filters may require more frequent changes and does not last as long as the capacity states.
Treatment recommendations for grains of hardness Phosphates:
Phosphates: <= 7
Water softener (ion exchange): <= 12
NAC blend: <= 18
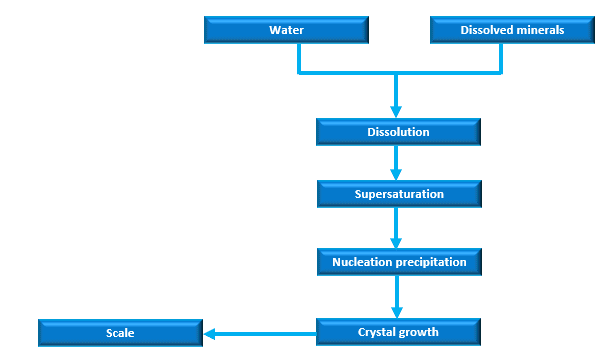
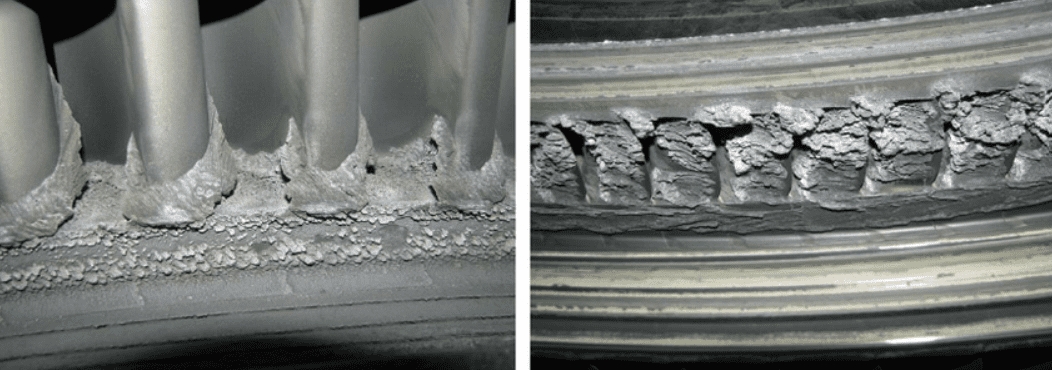
There is a lot of science behind scale formation, but here are the basics:
Dissolution
Supersaturation
The point of crystallization is the main cause of scale deposition. High concentration of dissolved solids that cannot dissolve in the solution.
Causes of Supersaturation
- Temperature increase
- pH increase
- Pressure decrease
- Flow velocity decrease
Nucleation precipitation
The electrostatic attraction between the metal surface and scale-causing minerals
Crystal growth
Crystals formed in one part of a system and carried to another part are less abundant than those formed on site.

Hard water and coffee extraction
Did you know?
Observations from different sources cite a tendency for “over-extracted flavor” at high levels of total hardness (> 250 ppm or 15 grains) and, conversely, a trend for “under-extracted flavor” at low levels of total hardness (< 40 ppm or 2 grains).
However, experimental measurements suggest that the impact of total hardness on the overall extraction efficiency is insignificant within reasonable variations in total hardness levels (20–250 ppm or 1–15 grains).
Municipality treatment
Chlorine was first used in the United States as a major disinfectant in 1908 in Jersey City, New Jersey. Chlorine use became more common in the following decades, and by 1995, about 64% of all community water systems in the United States used chlorine to disinfect their water.
Chloramines (chlorine and ammonia) have been used as drinking water disinfectants in the United States in places like Cleveland, Ohio, Springfield, Illinois, and Lansing, Michigan, since 1929. In 1998, an EPA survey estimated that 68 million Americans were drinking water disinfected with chloramine. Several major U.S. cities, such as Philadelphia, San Francisco, Tampa Bay, and Washington, D.C., use chloramines to disinfect drinking water.
Research shows that chloramine and chlorineboth have benefits and drawbacks.
Chlorine is a highly effective method of disinfection. However, while in the pipes, it produces small amounts of chemicals (called “disinfection by-products") if the source water has higher levels of dirt or germs that may react with chlorine.
Chlorine is also used up quickly in water systems. Sometimes, there is not enough chlorineleft to kill germs in the water by the time it reaches the end of the pipes. Chloramine can last longer in the water pipes and produce fewer disinfection by-products.
Unlike chlorine, chloramine cannot be removed by letting water sit out for a few days, nor can it be removed with regular coconut shell carbon. To effectively remove Chloramines, catalytic carbon should be used. This is because Chloramines are extremely corrosive to pipes. Aging water systems can lead to copper pipe corrosion, which damages your pipes and contaminates your water. When copper pipes corrode and get damaged, it can result in bacterial growth and attract harmful chemicals such as lead, zinc, and copper.
According to EPA data, over one in five American homes use drinking water that has been treated with chloramines. Chloramines have been linked to pinhole leaks in copper pipes, which, while seemingly small, can lead to big repair issues. Due to the tiny size of pinhole leaks, these leaks can go undetected for a prolonged period of time. This can not only lead to gallons of wasted water but also contribute to water damage and mildew.


Municipality treatment
Orthophosphate is a common corrosion inhibitor used by water suppliers to prevent lead pipes from leaching. When orthophosphate water treatment is added to a water source, it reacts with lead to create a mineral-like crust inside the lead pipe. This crust acts as a coating that prevents further lead corrosion.
The use of orthophosphate treatment in drinking water became popularized in 2001, during the lead crisis in Washington, D.C. Lead contamination in many cities, including D.C. and Flint, occurs when a city’s water becomes more corrosive, which can allow for lead from pipes to leach into the drinking water supply.
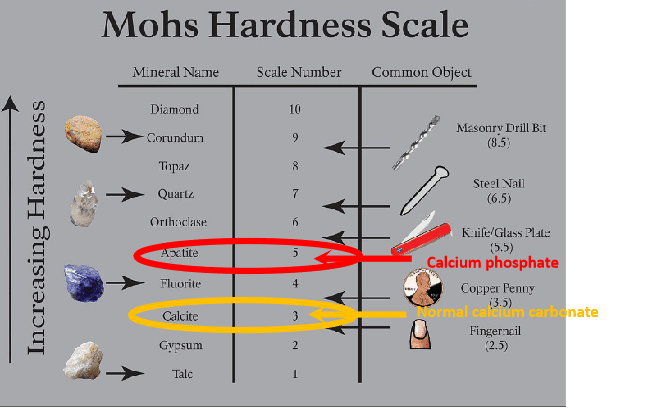
Calcium phosphate scale can form on surfaces that meet water containing high levels of calcium and phosphate ions¹. The formation of the calcium phosphate scale is influenced by several factors, such as temperature, pH, and the concentration of calcium and phosphate ions².
(1) Calcium Phosphate Scale Formation from Simulated Milk Ultrafiltrate Solutions - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S096030850270326X.
(2) Mechanisms of Scale Formation and Inhibition - ScienceDirect. https://www.sciencedirect.com/science/article/pii/B9780444632289000036
Calcium phosphate is no ordinary scale (calcium carbonate); it is both physically harder and harder to remove when it forms. On the Moh’s 1-10 scale of mineral hardness, normal calcium carbonate (calcite) is a 3, and calcium phosphate is a 5.
Suspended particles cannot be completely removed by plain settling. Large, heavy particles settle out readily, but smaller and lighter particles settle very slowly or, in some cases, do not settle at all. Because of this, the sedimentation step is usually preceded by a chemical process known as coagulation. Chemicals (coagulants) are added to the water to bring the non-settling particles together into larger, heavier masses of solids called floc. Aluminum sulfate (alum) is the most common coagulant used for water purification.
After the flash mix, a longer period of gentle agitation is needed to promote particle collisions and enhance the growth of floc. This gentle agitation, or slow mixing, is called flocculation.
Municipalities use over 60% of the aluminum sulfate produced in the US for applications such as water treatment and wastewater treatment to get particulate-free and clear water.
Calcium Sulfate (CaSO₄) is an insoluble scale. Like the phosphate scale, it doesn’t dissolve with an acid.
How do you tell the difference between the temporary (Calcium carbonate) scale and the permanent (Calcium phosphate) scale?
Pour an acid (vinegar or lemon juice) on the scale; use a brush (if needed). If it dissolves, it’s temporary. If not, it’s permanent.
Silica scaling is a large problem, especially at high temperatures, because fluid forms in contact with rocks.
Where Silicium is the basic rock-forming element, the solubility of silica decreases with the temperature decreases. This type of scale is extremely difficult to remove. The Silica scale is considered a permanent scale.
Contact Time
Contact time requirements depend on the equipment. This can easily be determined by reviewing the equipment installation manual or user guide. If not readily found, contact the manufacturer and ask for the peak gallons per minute (GPM) for the equipment.
Unfortunately, many times, this is not considered, and water treatment systems are undersized for the actual demand of the equipment that is needed for effective treatment.



